The intermolecular bonding forces between ether molecules are comparably weak which results in low boiling points / high vapour pressures. And because when you "light a fluid" you always in fact just light it's vapours above the surface, higher vapour pressures result in higher flammability.Extremely flammable. Vapour/air mixtures are explosive. NO open flames, NO sparks and NO smoking. NO contact with hot surfaces.Previously used as an anesthetic, Diethyl ether is an organic compound used primarily as a solvent. This colorless, highly volatile chemical has a pungent, yet somewhat sweet odor. Due to its extreme flammability and other potential hazards, diethyl ether should be stored and handled with extreme care in the workplace.
What happens when diethyl ether is exposed to air : Diethyl ether is highly flammable and explosive when mixed with air (explosive limits: 1.9–48.0%), the tiny spark from switching on a light could ignite it.
Why is diethyl ether so volatile
But in the case of ether, no such bonding is present, and molecules are held together by weak intermolecular forces. Hence, due to the presence of weak intermolecular forces ethers are volatile in nature.
Why did we stop using diethyl ether : While ether was effective as an anesthetic, it did have its shortcomings. It was highly flammable, and once it was released into the air, it could easily cause explosions. As well as this, patients often felt a chocking sensation, and because the onset could last up to 15 minutes, the patients had to be held down.
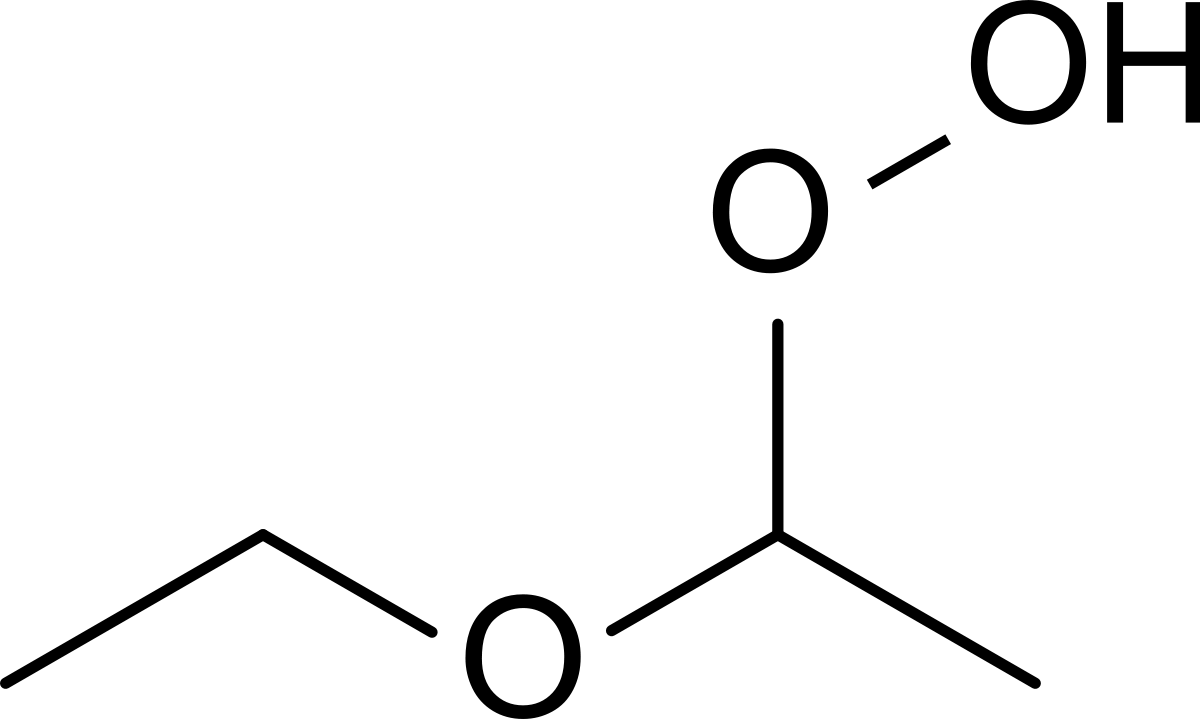
Ethers absorb and react with oxygen from the air, in the presence of light, forming unstable peroxides that can detonate with extreme violence when they become concentrated through evaporation or distillation and disturbed by heat, shock or friction.
Diethyl ether on heating with conc HI gives two moles of : ethyl iodide. ethanol.
Is diethyl ether a drug
The anesthetic and intoxicating effects of ether have made it a recreational drug. Diethyl ether in anesthetic dosage is an inhalant which has a long history of recreational use. One disadvantage is the high flammability, especially in conjunction with oxygen.A liquid with weak intermolecular forces evaporates more easily and has a higher vapor pressure. A liquid with stronger intermolecular forces does not evaporate easily, and thus has a lower vapor pressure. For example, diethyl ether is a nonpolar liquid with weak dispersion forces.* Diethyl Ether is on the Hazardous Substance List because it is regulated by OSHA and cited by ACGIH, DOT, DEP, NFPA and EPA. * This chemical is on the Special Health Hazard Substance List because it is FLAMMABLE.
In the 21st century, ether is rarely used. The use of flammable ether was displaced by nonflammable fluorinated hydrocarbon anesthetics. Halothane was the first such anesthetic developed and other currently used inhaled anesthetics, such as isoflurane, desflurane, and sevoflurane, are halogenated ethers.
Why does ether catch fire : An ether reacts with air to form peroxides which are highly unstable, hazardous in nature and catch fire easily.
Why is ether so volatile : Hence, due to the presence of weak intermolecular forces ethers are volatile in nature.
Can you boil diethyl ether
A bit more interesting, di ethylether, boils at 34 degrees Celsius.
The correct option is B Ethyl Alcohol
STATEMENT-1 : Diethyl ether when boiled with water gives ethyl alcohol.Diethyl ether was found to have undesirable side effects, such as post-anesthetic nausea and vomiting. Modern anesthetic agents reduce these side effects. Prior to 2005, it was on the World Health Organization's List of Essential Medicines for use as an anesthetic.
How does ether make you feel : Effects. The effects of ether intoxication are similar to those of alcohol intoxication, but more potent. Also, due to NMDA antagonism, the user may experience distorted thinking, euphoria, and visual and auditory hallucinations at higher doses.